Upcoming Virtual dSTORM Training – Coming January 29, 2026 register now>
Understanding pathogens with super-resolution microscopy
Pathogens Hub
Characterizing pathogens
Infectious diseases are caused by pathogenic microorganisms such as bacteria, viruses, fungi or prions. Thanks to scientific advances in microbiology, host-pathogen interactions, and drug development, many of these infections can now be controlled or cured. Despite great efforts and the development of advanced technologies to investigate infectious agents, there are still many open questions regarding pathogen life cycles, how they cause disease and how can they be eradicated.
Chapter 1
Pathogens in greater detail
One of the biggest challenges in pathogen research is their size; in the range of micrometers down to nanometers. Visualizing their propagation and interaction with the host can be extremely challenging. Advances in having better tools to observe and study the architecture, interactions, and drug-targeting effects on such pathogenic agents will bring scientists closer to finding more efficient ways of treating and eradicating devastating infectious diseases.
Conventional microscopy techniques, such as confocal microscopy, are limited by the diffraction of light, with individual molecules only resolved at a spatial resolution of 200 nm, at best. In contrast, single-molecule localization microscopy (SMLM) techniques such as dSTORM or PALM allow the visualization of specific biomarkers (proteins, antibodies, nucleic acids, and other biomolecules), their distribution and interactions on biologically relevant scales, achieving resolutions of 20 nm.
This means that super-resolution microscopy can be used by virologists to directly estimate the size of viral particles on a glass surface. The added advantage lies in the fact that by specifically labeling nucleic acid molecules or capsid proteins, we can look closer at the composition of viral particles using multiple colors, and learn the spatial relation between particle components.
An example of the benefit of super-resolution imaging with dSTORM microscopy is presented viral particle imaging case study, where the surface glycoprotein and cellular host factors incorporated into reconstructed HIV particles were examined with a resolution reaching 20 nm that allowed the study of particle sizes and spatial distribution of co-factors.
Super-resolution microscopy has also been used for research on sub-viral particles of pathogenic viruses. One of the most challenging aspects of studying the architecture of viral particles is their size; in conventional light microscopes, they appear as a diffraction limited structures, where dimensions and structural arrangements cannot be measured accurately.
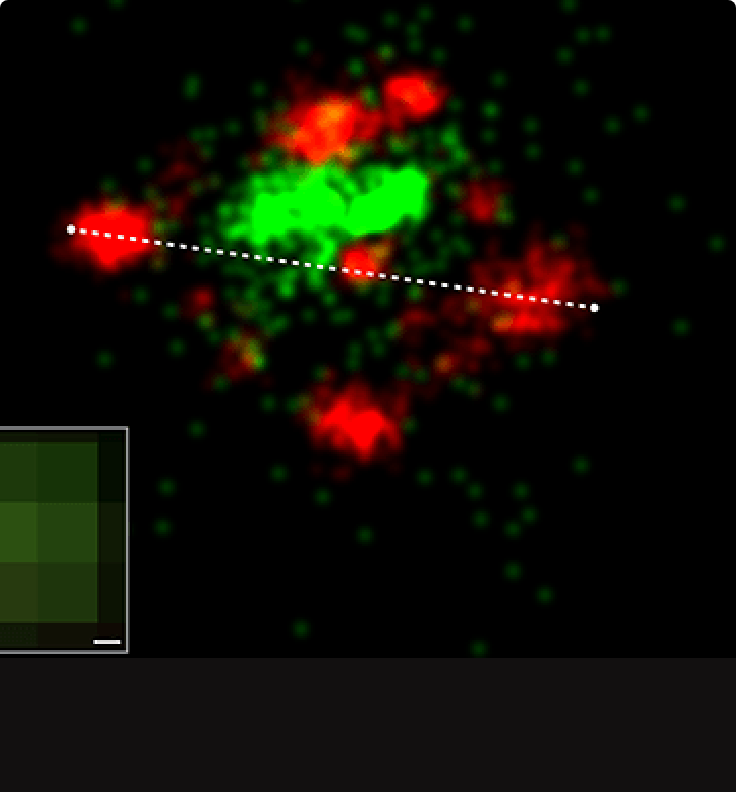
Figures A & B show nucleocapsid-like assembly of Marburg virus, acquired by dSTORM. The observed width of the nucleocapsid filament of the subviral particle was approximately 105 nm (panel A). This data also revealed that the structural orientation of the nucleocapsid has a corkscrew-type shape (panel B), as has been seen before using cryoEM studies (Bharat et al., 2012).
Implementation of SMLM techniques such as dSTORM or PALM to pathogen research is invaluable, as these techniques can provide a better understanding of protein organization throughout the pathogen’s life cycle, offer quantitative data of the factors necessary for virulence, and give insight into key interactions necessary for both pathogenicity and therapeutic targeting. In the long-term, it will empower researchers to create new ways to prevent and treat infection.

The Marburg virus nucleoprotein, expressed in human hepatoma cells (HuH-7) was labeled with AF647 and imaged by dSTORM using BCubed buffer. Thanks to Prof. S. Becker, Dr O. Dolnik and Dr K. Grikscheit and the Team from Marburg University for sample preparation and insightful discussions.
Single-particle tracking
What do we mean by single particle? There is no simple answer, as both the size and the composition of the particle can vary greatly. Any combination of molecules that behave as one entity can be followed and analyzed – from individual viruses or vesicles to single molecules, in solution or intracellularly. Dual-color tracking is a powerful tool to identify single particles with high accuracy. By co-labeling two molecules of interest with spectrally distinct fluorophores, the dynamic behavior of each can be studied and quantified using the other as a comparison or reference point.
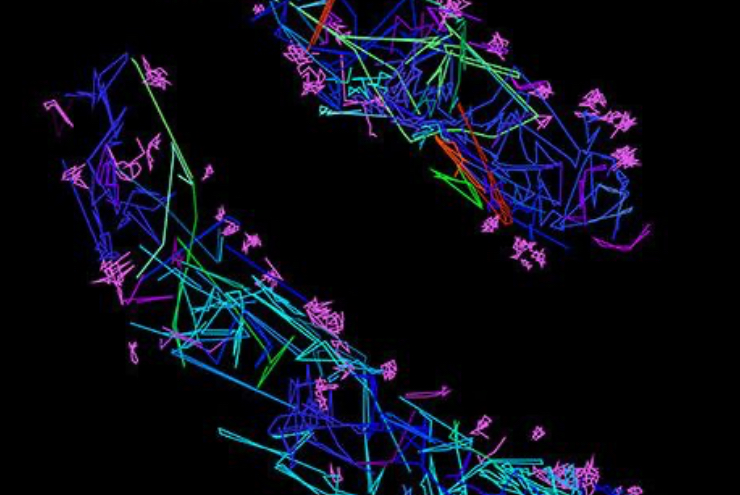
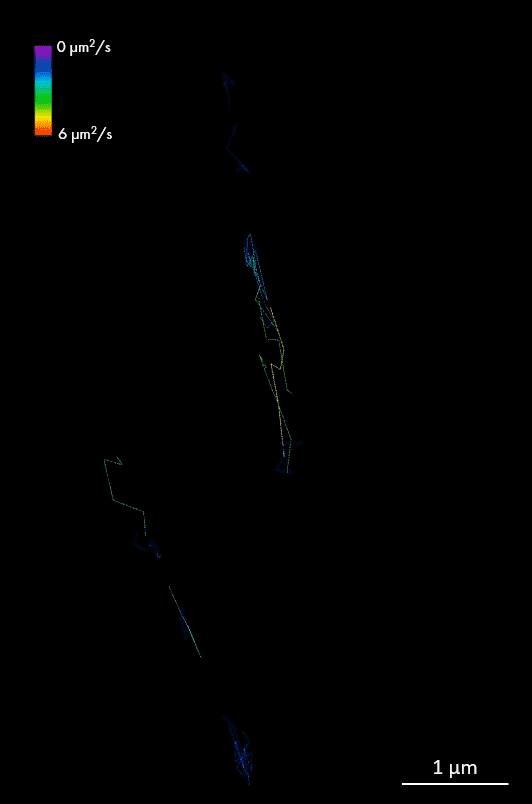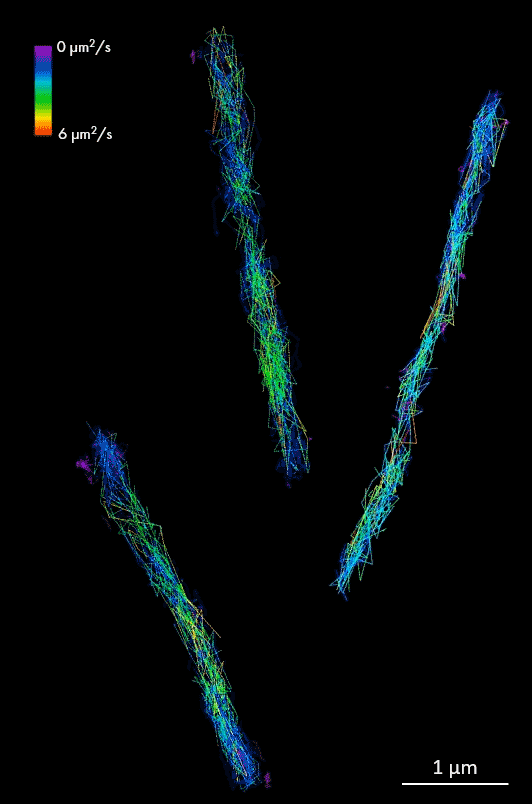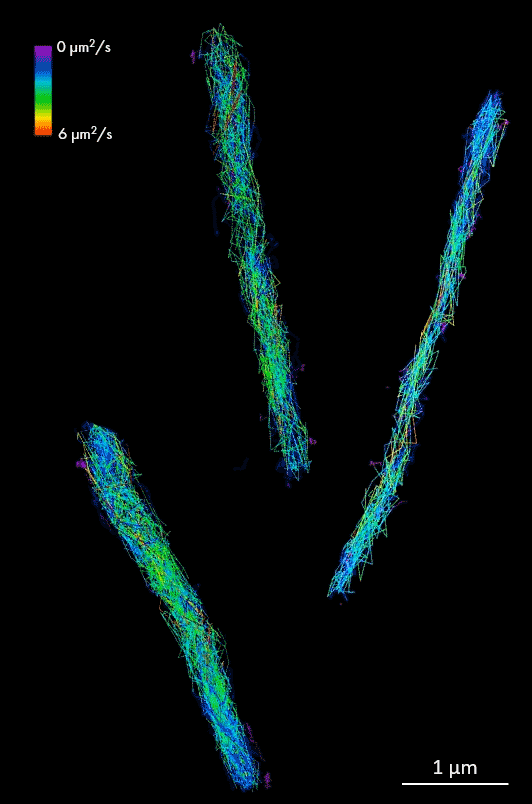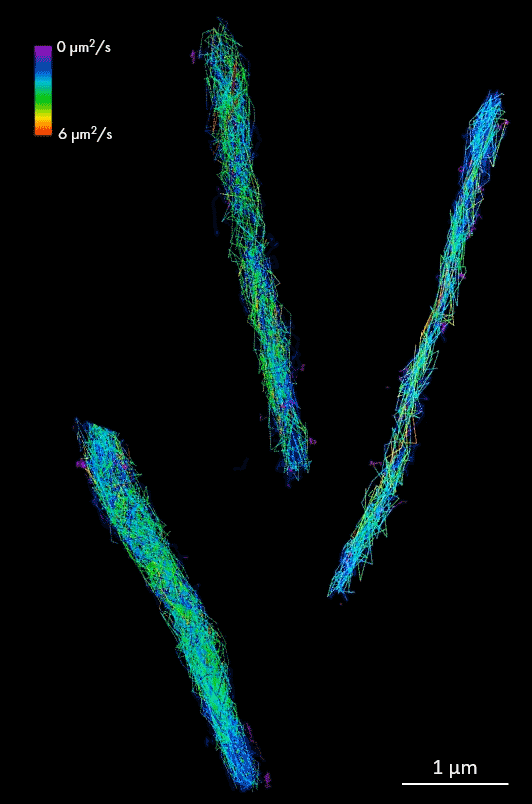
For larger pathogens, like bacteria and fungi, single-particle tracking enables characterization of the behavior of individual molecules, in respect to one another, cellular structures or chromosomal markers. An example is presented on the video showing single-particle tracking of T3SS protein (a molecular syringe, also known as “injectisome”, used by gram-negative bacteria to inject effector proteins into host cells) in bacteria. Single-particle tracking (SPT) is applied to describe how T3SS complexes are activated and regulated during the infection process, and how their function can be controlled or inhibited.
The T3SS protein is essential for virulence in many important human pathogens, including Salmonella, Shigella, and pathogenic Escherichia coli strains that cause several millions of deaths per year. It is also particularly important in hospital-acquired infections, for example, for Pseudomonas aeruginosa where it is associated with increased antibiotic resistance and severe disease symptoms. Thus, T3SS is an important target for anti-virulence therapeutics.
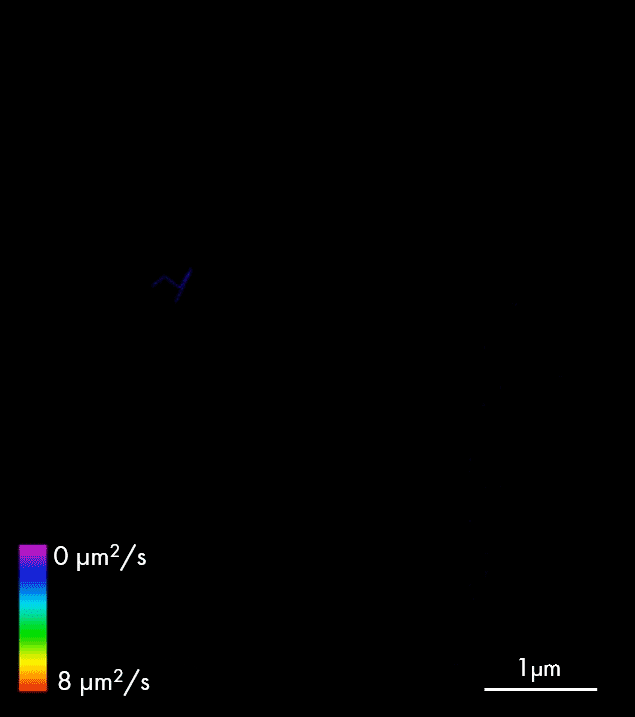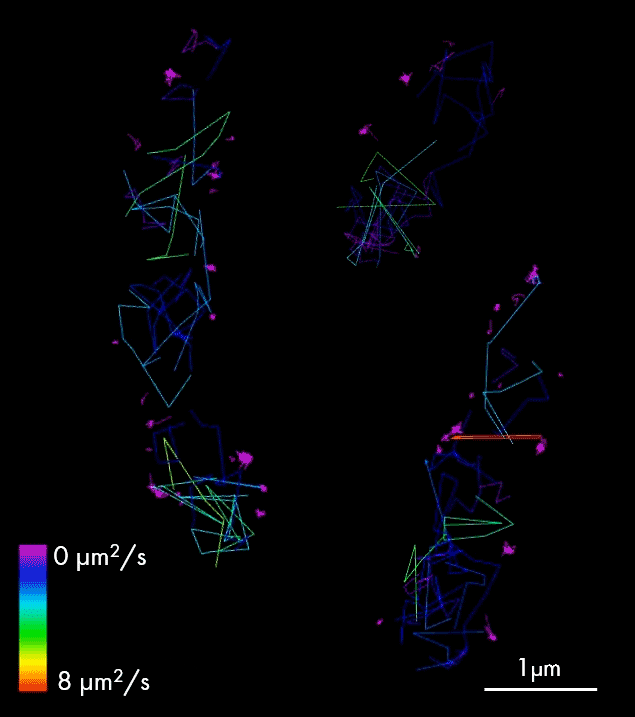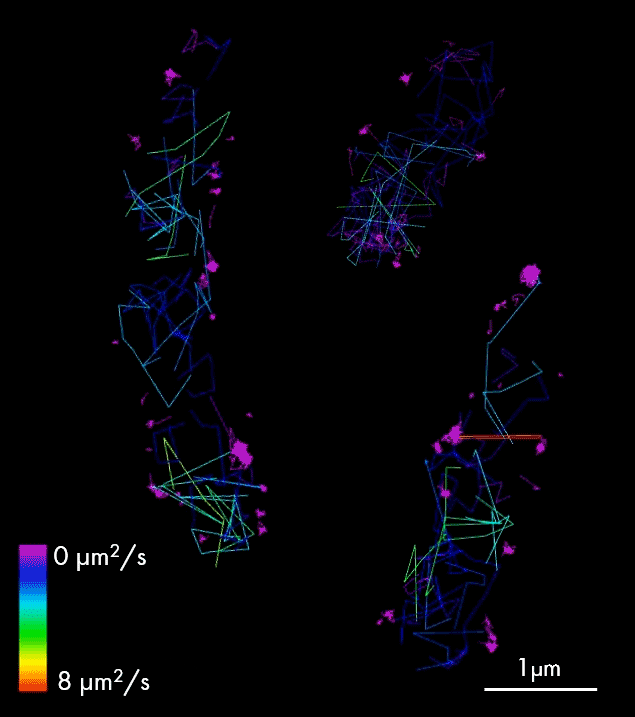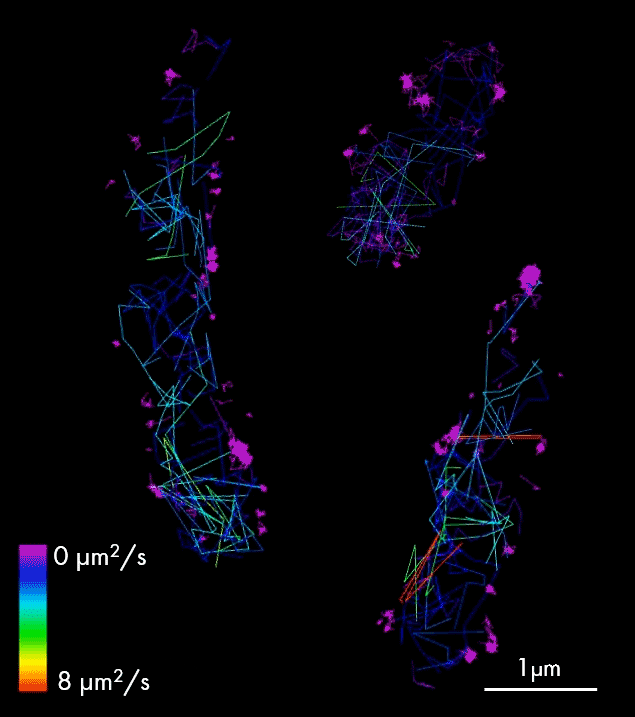
Single-particle tracking of T3SS-HALO stained with JF549 in live bacteria. Sample provided by Dr. A. Diepold, Max Planck Institute for Terrestrial Microbiology, Marburg, Germany.
Chapter 2
Host and Pathogen
From entire populations of infected individuals to a single molecule, infectious diseases and their pathogenicity all boil down to host-pathogen interactions. Macroscopic and microscopic tools to analyze the transmission of diseases and the mechanisms of immune system evasion ultimately provide a better context for infectious disease prevention and treatment protocols.
No matter what pathogen is being studied, in all cases, the different steps leading to the disease state are highly complex in nature and require the understanding of many converging and often competing signaling pathways.
Studying the dynamics of host-pathogen interactions
The interaction between the virus and the host cell is an essential part of any virus life cycle, as all viruses depend on their host to propagate. This is why it is extremely important to understand virus entry, replication within and release from the host cell. One of the challenges when studying these steps is that the viral interaction with the host occurs below the diffraction limit of conventional light microscopes.
By analyzing the motion of viral particles using single-particle tracking, we can understand the mechanisms of viral particles upon, for instance, drug treatment, and observe them in a living cell environment.
An excellent example of how single-particle tracking enables spatiotemporal dynamic localization of viral particles throughout their life cycle in Huh-7 cells can be seen in this viral particle case study, where some viral structures are able to move directionally, a process that is dependent on host actin cytoskeleton. Disruption of this viral-host interaction may lead to the generation of new drugs against Ebola infection.
In conclusion, by analyzing and quantifying the diffusion of key molecules, we are able to extract detailed information about specific biological events in real-time, such as binding, transport or compartmentalization. Combining super-resolution imaging with spatial information at the nanometer scale, we gain a deeper understanding of how different events are related, why certain inhibitors or therapeutic drugs may not be working, and what protein interactions are needed for a specific process to occur. In other words, correlating temporal and spatial information at this level of detail enables researchers to make important discoveries and develop new methods for tackling devastating infectious diseases that affect millions worldwide.

Tracking of Ebola nucleocapsid-like structures (NCLS-GFP) inside human liver Huh-7 cells.
Chapter 3
Pathogen Nanoimaging
Learning about disease mechanisms, potential vaccine targets and development of accurate therapeutics agents comes with multiple challenges. One of the crucial ones is combining complementary characterization techniques, such as super-resolution information with dynamic live-cell imaging of pathogens and their interactions with host cells.
Quantitative characterization of pathogens made simple
Pathogenesis or infection stages take place at different time-scales; from a few seconds to hours. It is therefore essential to be able to follow these processes in real-time, without perturbing the biological system under investigation. From a technical standpoint, this requires a microscope that is both compatible with long-term live-cell imaging and meets the needs for single-molecule sensitivity.
Additionally, working with disease-causing agents mandates strict safety precautions. To reduce the risk of human exposure to infectious agents, scientists need to work in controlled, and often very challenging environments, such as biosafety cabinets, and facilities with higher levels of containment. Given these constraints, there is a requirement for tools that not only are small enough to work in confined environments but also are easy to operate by the primary user without the need for back-up staff to be cleared to work in highly controlled environments. By providing the best tools in these environments, researchers can ensure the fight to deliver diagnostics, vaccines and antivirals world-wide continues at the fastest rate.
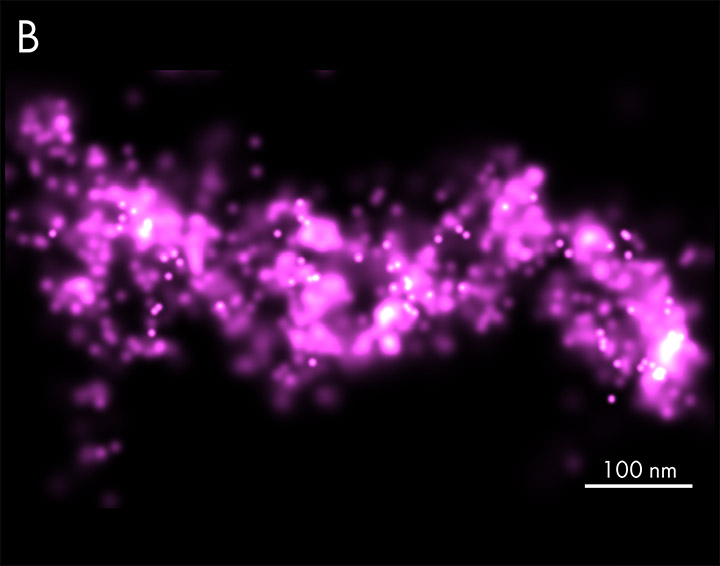
HALO-labeled FtsK protein bound to TMR ligand, single-particle tracking in Mycobacterium smegmatis cells. Samples provided by Zakrzewska-Czerwinska lab, Wroclaw University, Poland
The Nanoimager: fit to perform, developed to fit
The unprecedented stability and compact design of the Nanoimager enables super-resolution microscopy and different imaging modes within environments where space is limited and safety is paramount. ONI has designed the Nanoimager to be a turn-key solution, combining the ultimate techniques for super-resolution imaging into an intuitive, compact, and stable system. ONI’s dedicated software, NimOS, allows real-time localization rendering and subsequent quantification of viral particle size, diffusion rates and concentration in a fast and user-friendly manner. In combination with its unique capability to minimize vibrations, the Nanoimager is an ideal platform to fit in a biosafety cabinet, where researchers can carry out single-molecule and super-resolution experiments on infectious agents with an ease-of-mind – read more about how we do imaging in biosafety cabinets.
The Nanoimager is one of the most sensitive microscopes available due to its unique closed-form design. It supports super-resolution microscopy as well as live-cell imaging in the most accessible format ever designed. Using this platform, pathogens can be characterized with a resolution reaching 20 nm and subsequently imaged to measure their behavior in host cells. Up to four molecular species can be measured in the same sample using different fluorescent markers.
Furthermore, compatibility with microfluidics supports drug and vaccine testing. Microfluidic technology enables miniaturization of a fluidic environment and enables in vivo testing of compounds mode of action, dosage and efficiency at both a single-cell and populational level.
Taken together, ONI has set out on a mission to provide scientists with the necessary tools to develop better diagnostics, vaccines and antiviral treatments through making studies on infectious pathogens easy and accessible for all. Our application scientists have extensive experience in the area and are happy to give tips on sample preparation and help in choosing the best imaging techniques to make the most out of your ideas and samples. If you want to discuss your specific research questions with an application scientists then please get in touch by filling the form below.