Upcoming Webinar – Automated DNA-PAINT and Exchange Imaging with Aplo Flow Fluidics register now>
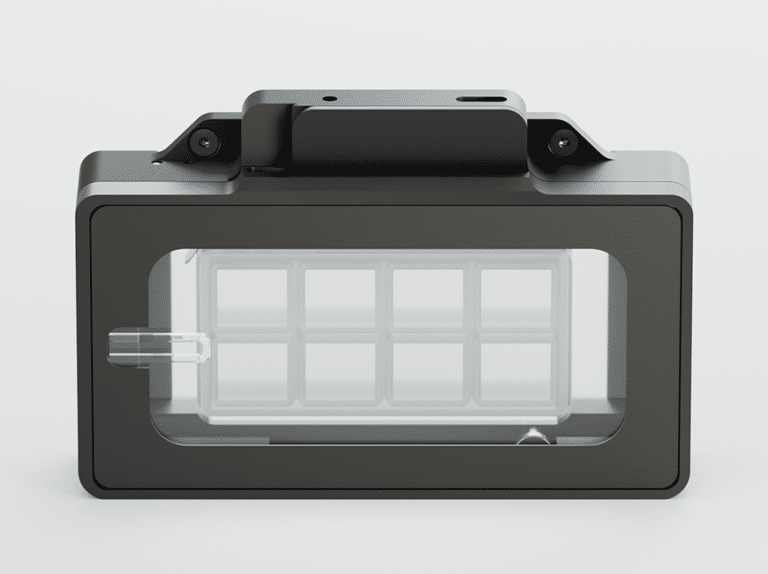
Nanoimager
A benchtop super-resolution microscope for your imaging needs

Key features
Nanoimager


View our virtual demo
Investigate your sample using different imaging technologies

dSTORM
Direct Stochastic Optical Reconstruction Microscopy (dSTORM) relies on the stochastic activation of single fluorophores that blink from “off” to “on”, and quickly back to “off”. The process is repeated many times, activating just one molecule within a diffraction-limited region at a time.
Best for: very high resolution, molecular distributions in fixed cells
Requires: tagging with compatible organic dyes, high brightness and blinking buffer

PALM
Photoactivated Localization Microscopy (PALM) uses photoactivatable fluorophores to resolve spatial details of tightly packed molecules. Once activated, fluorophores emit for a short period but eventually bleach. The laser stochastically activates fluorophores until all have emitted.
Best for: protein dynamics, organization and quantification, in fixed and live samples
Requires: genetic encoding using photoswitchable fluorescent proteins, low brightness
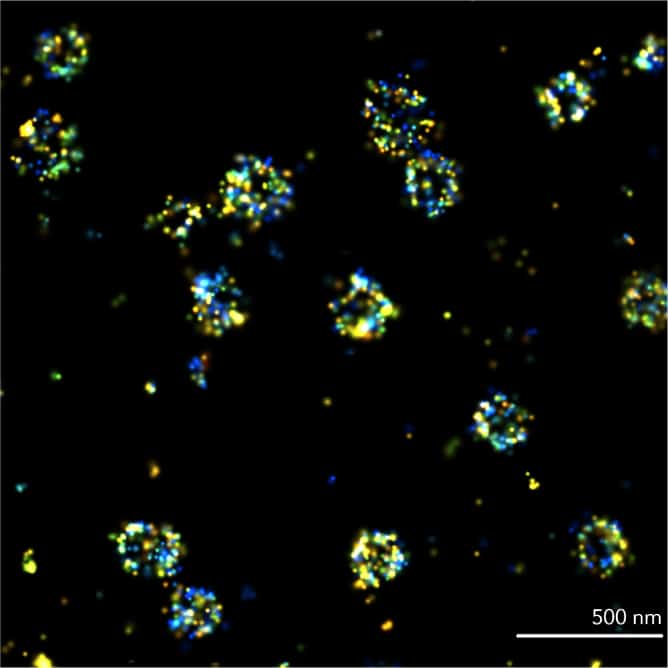
PAINT
In Point Accumulation for Imaging in Nanoscale Topography (PAINT), single-molecule localizations are achieved using transient binding of fluorophores to targeted proteins. PAINT is commonly performed with DNA strands < 10 nt. Like other SMLM techniques, it can reach spatial resolutions of 20 nm.
Best for: biomolecular organization and quantification, in fixed samples
Requires: specific transient binding molecules linked to fluorescent dyes

Single-particle tracking
Single-Particle Tracking (SPT) allows the motion of individual particles to be followed
in vitro or in living cells, to obtain information on their dynamic behavior over time. Trajectories can be extracted with a resolution of up to milliseconds.
Best for: protein dynamics and tracking, in live samples or in solution
Requires: genetic encoding using photoswitchable fluorescent proteins, low brightness, low tagging density.
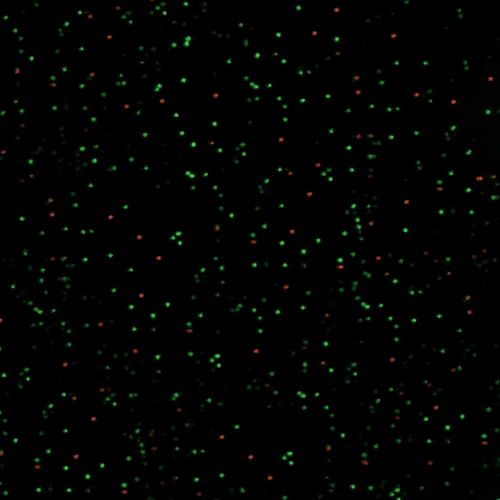
Single – molecule FRET (smFRET)
During single-molecule Förster Resonance Energy Transfer (smFRET), the emission energy of a donor fluorophore is transferred to an acceptor, which subsequently fluoresces when in close proximity. It enables distances between single molecules to be measured at a scale of 1-10 nanometers.
Best for: very high resolution, molecular distributions in fixed cells
Requires: donor and acceptor fluorophores with partially overlapping emission-absorption spectra.
Adjust the illumination angle for your sample
A parallel beam of light is passed directly through the sample, maximizing illumination. Also referred to as widefield microscopy, it is particularly useful for imaging thick samples, over 10 µm deep. The intense illumination and excitation of molecules outside the focal plane can produce a high background signal.
The beam of light is both highly inclined and also incredibly thin. This has the effect of increasing the image intensity, while at the same time reducing the background noise. The signal to background noise ratio can be up to eight times greater than standard. It also allows for an imaging depth of up to 10 µm, unlike TIRF that is limited to imaging the surface. HILO can be useful when imaging tissues, nuclei or other cell material that sit beyond the membrane.
Only excites fluorophores on the surface of molecules in the first 200 nm, so other fluorophores are not excited and do not emit light. As a result, background noise is significantly reduced. This improvement means that TIRF has become a leading technique in the study of single molecules. It is useful for samples with fine structures, sitting close to the coverslip, as well as for studying molecules attached to a surface or on a membrane.
Stage Top Incubator
The Stage Top Incubator is a practical addition for extending live cell studies with the Nanoimager. It provides reliable control over temperature, CO2, and humidity, which are crucial for keeping live samples in optimal conditions.
Designed to work seamlessly with the Nanoimager, the incubator is easy to set up and doesn’t require any hardware changes. It can accommodate both 35 mm dishes and standard microscopy slides, making it a flexible tool for various lab needs.
FAQs
Yes. The Nanoimager is capable of live imaging and multi-position acquisitions through whole microscope unit heating up to 42°C, external port compatible with CO₂ feed and software support for time-lapse, multi-position and Z-stack.
Yes. The Nanoimager has approximately the same footprint as a piece of A4 paper, with dimensions of 21 cm (w) x 15 cm (h) x 21 cm (d). The light engine is 21 cm x 42 cm x 45 cm and they are attached to each other by a 1.5 m cable containing crucial optical parts.
55kg for the entire system.