Introducing: ONI’s Next-Generation End-to-End Solution for EV Research Learn More
Simple Overview
Immunofluorescence
What is immunofluorescence?
Immunofluorescence (in short, IF) is a method in biology that relies on the use of antibodies chemically labeled with fluorescent dyes to visualize molecules under a light microscope. For a successful IF staining, it is crucial to have a good antibody that will specifically detect the antigen(s) within the molecule of interest.
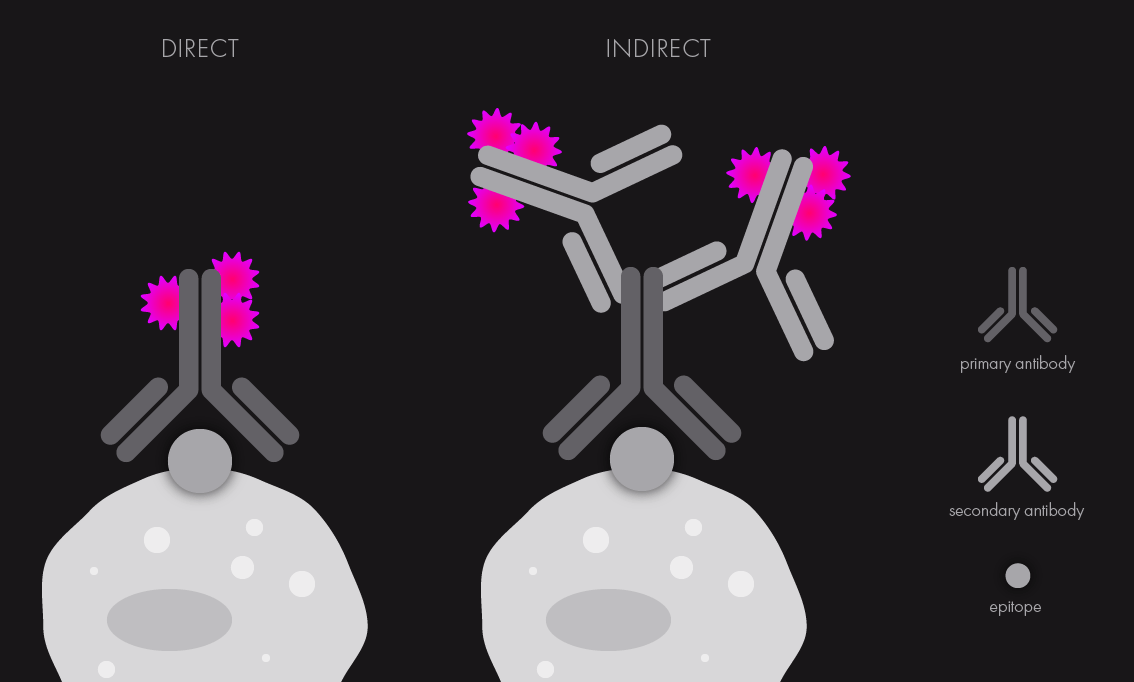
This antibody (often called primary antibody) is covalently linked to a fluorescence dye or will be subsequently bound by a specific second antibody of a different origin (called secondary antibody), which will be labeled with the desired fluorescent dye. The first instance is known as direct immunofluorescence, while the second case is known as indirect IF. Both approaches allow for differently-labeled antigens to be visualized using antibodies attached to fluorophores with distinct emission spectra, meaning that they can be imaged using separate laser lines.
Why immunofluorescence is useful
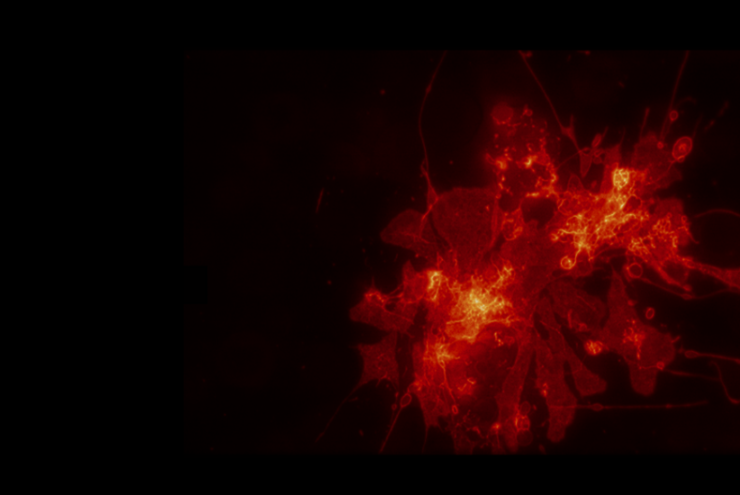
Immunofluorescence is commonly used in molecular and cell biology labs as a robust and simple method to reliably localize molecules on a wide range of fixed cells or tissues. IF staining offers the unique possibility of revealing molecules in their “native” state, minimizing potential perturbations of protein conformation, localization and/or function, which can occur when using fluorescence protein tagging.
Similarly, immunofluorescence is highly informative when studying steady state or endogenous protein levels and localization – fluorescence proteins, on the other hand, can sometimes multimerize or affect the kinetics and expression levels of molecules.
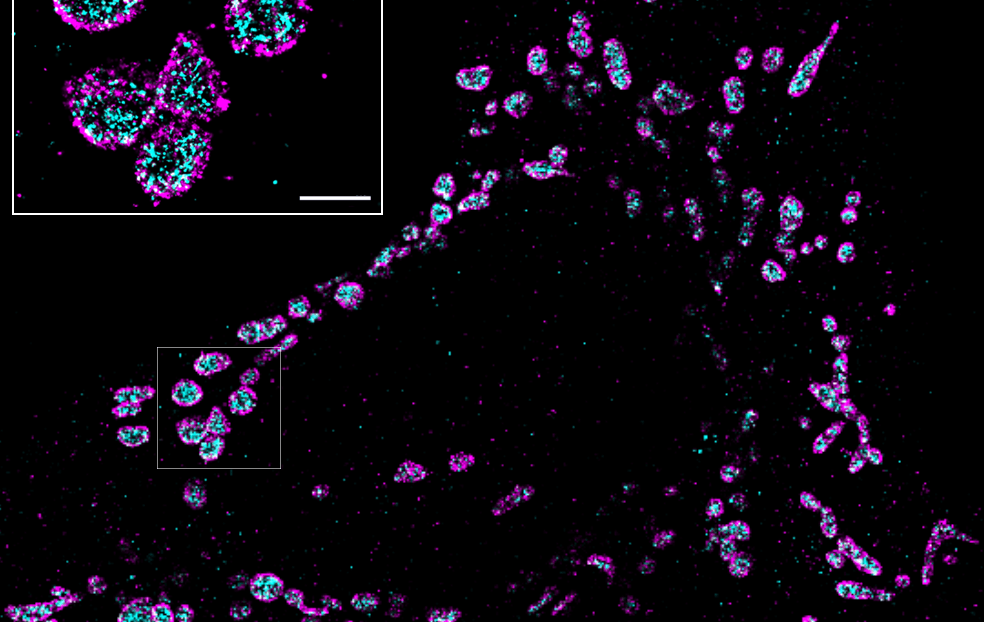
Can I image immunofluorescence samples on the Nanoimager?
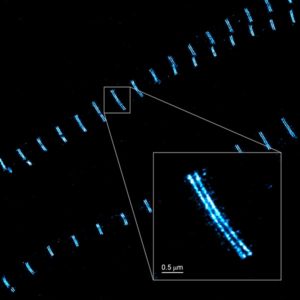
The Nanoimager significantly enhances the resolution of fluorescence microscopy to up to 20 nm with different super-resolution techniques, including dSTORM, PALM, single-particle tracking and smFRET. Samples stained using immunofluorescence methods, can be imaged in a fast and automated way using our integrated system, which includes the dedicated NimOS software for image analysis.
The Nanoimager can capture two fluorophores simultaneously (with four different colors) and offers three different illumination modes, epifluorescence, TIRF and HILO, improving signal to noise ratio depending on the thickness of the sample. It also provides unrivalled stability, and thanks to its compact design, it can be used in any lab environment without needing an optical table or designated dark room.
Read our 9 tips on how to optimize your immunofluorescence staining blog post. Learn more about the Nanoimager and the different applications it is being used for.